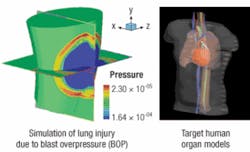
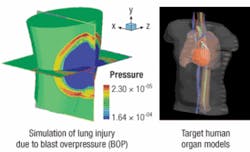
Nanotechnology delivers military power
Scientists are using nanotechnology to create materials with properties that will revolutionize military technology, from processors to display screens and body armor to air filters.
By Ben Ames
Army leaders are looking for a 21st century battlesuit. The lycra-tight clothing must stop bullets, detect chemical and biological agents, monitor a wounded soldier’s vital signs, administer basic first aid, and communicate with headquarters.
One approach could provide answers for all those challenges-nanotechnology.
In March 2002, Army planners granted $50 million to the Massachusetts Institute of Technology in Cambridge, Mass., to create the Institute for Soldier Nanotechnologies. Across the country, military research dollars are flowing to nanotechnology laboratories, as engineers find ways to build more-efficient batteries, more-powerful fuel cells, more-receptive solar cells, more-affordable titanium metal, and more-sensitive chemical and biological agent detectors.
Nanotechnology is no longer a dream discipline for future research-it is creating products for specific applications today.
The soldier suit
At MIT’s Institute for Soldier Nanotechnologies (ISN), researchers collaborate with engineers at Dupont, Partners Healthcare, and Raytheon. Together, they combine expertise in chemistry, medicine, and mechanical engineering-and wrap it all up in nanotechnology-based materials. The result could transform today’s cotton and nylon fatigues and bulky equipment belts into lightweight battlesuits that provide a suite of integrated systems.
ISN researchers divide their projects into seven teams:
- Team 1: energy absorbing materials
- Team 2: mechanically active materials and devices
- Team 3: sensing and counteraction
- Team 4: biomaterials and nanodevices for soldier medical technology
- Team 5: processing and characterization
- Team 6: modeling and simulation of materials and processes
- Team 7: systems design, hardening, and integration
Team 1 engineers are developing nanomaterials that will be part of the future soldier’s uniform, helmet, and gloves. Some scientists are studying new molecular architectures for ultrastrong energy-absorbing polymers, while others study nanostructured materials for ballistic and blast protection.
Team 3 engineers are seeking ways to detect and respond to chemical and biological threats. Their projects include microbicidal, antiviral, and antisporal fabrics; infrared detection systems for optical sensing; nanoparticle assemblies as chemical toxin deactivation coatings; and fluorescent sensing technologies for selective detection of chemical warfare agents.
Team 4 researchers are working with doctors to use nanotechnology to automatically treat soldier medical conditions like hemorrhage, fracture, and infection.
Team 7 designers are creating battlesuit cooling systems, and a nano-enabled, multifunctional soldier glove with microbicidal coating. (For more information, see web.mit.edu/isn).
How small is nano?
Every November, military planners at the U.S. Army’s Picatinny Arsenal, N.J., invite nanomaterial producers to describe their wares. In 2004, leaders from QuantumSphere Inc. unveiled their new factory in Costa Mesa, Calif. The company can create 2,500 pounds per month of nanoaluminum and nanonickel powders to make better propellants and munitions.
Workers in the new factory can create consistently sized particles, each coated with an oxide shell; uncoated nanometal powders will automatically weld to each other. Each nanonickel powder particle is 8 to 10 nanometers in diameter-about 10 atoms across, says Doug Carpenter, QuantumSphere’s chief science officer and cofounder.
In the past, factories created nanopowders by smashing large blocks of material into tiny bits, yet QuantumSphere’s new facility evaporates the original material into atomic particles, condenses it, and mixes it with oxygen gas to create the coating.
Military applications include more than munitions. Military designers are trying to replace platinum as the standard catalyst in fuel cells. Expensive and heavy, platinum loses efficiency as it gets carbonized during temperature cycles, he says.
QuantumSphere’s powder could be a solution. The company’s engineers mix those tiny particles into a substrate to create membranes and filters that are thin and durable. Such reinforced materials can survive many more thermal cycles than standard membranes, and they can serve as drop-in replacements for platinum in either solid oxide or PEM (proton exchange membrane) fuel cells.
“Military guys carry more batteries than ammunition or food. I’d like the guy to have more beans and rice and bullets, and still keep the same electric power,” Carpenter says.
Nanomaterials are still more expensive to prepare than metal ore, but platinum is so expensive - 1,700 times the cost of nickel-that nanonickel still ends up being one-eighth the cost.
Other applications for nanonickel and nanoaluminum alloys could be strong, temperature-resistant materials for piping, airframes, and wing edges. They are also good at shielding electromagnetic interference (EMI) because ferrous nanopowders are magnetic.
“It’s hard to imagine that a particle with just 300 to 1,000 atoms can maintain its magnetism, but highly magnetic materials are important for shielding,” Carpenter says. They can also act as sensors for medical toxins like anthrax and sarin. Those super-strong membranes can also be used to filter toxins out of a soldier’s water and air supplies.
Nanoparticles to charge batteries
The 21st century soldier is weighed down with batteries for electric laptop computers, handheld computers, night-vision goggles, and optoelectronic rifle sights. That load could soon be reduced, thanks to anodes made of nanoparticle-size crystals. Soldiers could recharge the new batteries in a fraction of the time, and thus could carry fewer batteries on the battlefield.
“Anodes with nanoparticles could be charged an order of magnitude faster than macro-size particles, if there’s plenty of lithium ion for their huge surface area,” says Ken Lyon, vice president for business development at Altair Nanomaterials Inc. in Reno, Nev.
The technology would also be crucial in electric engines, whether they are driving commercial automobiles or unmanned ground vehicles.
In experiments with the new anodes, researchers with Telcordia Technologies Inc. in Piscataway, N.J., and Rutgers University in New Brunswick, N.J., charged empty batteries to 80 percent capacity in just six seconds, he says. Altair built anodes for that research under a grant from the U.S. National Science Foundation.
Engineers still have some work to do. The new design has less storage capacity than standard lithium ion batteries, but is comparable to nickel metal hydride and nickel cadmium batteries. The latter is the type used in most cell phones, PDAs, and laptops.
If they succeed, the new batteries would be remarkable. Used in a portable power tool, the new design would cost the same, offer three to four times more power per unit of weight, recharge 20 times as often, and gain a full charge in six minutes instead of two hours. Over its lifetime, such a battery could recharge 10,000 to 20,000 times, compared to the current standard of 600 to 700, Lyon says.
Altair engineers grow their crystals from lithium titanate, each one 20 to 25 nanometers in size. At that size, the crystals are strong enough not to break when battery makers force lithium into them-the chemical process that recharges a battery.
“We can put the dog in the doghouse and it stays there,” Lyon says.
Nanoscale batteries pick up a charge faster than other batteries because they offer much more surface area to touch the lithium ion. Altair crystals have surface area of 100 square meters per gram, compared to two or three meters per gram for standard anodes.
Altair researchers can also use their nanocrystals to create titanium more cheaply. Titanium is strong and lightweight, but its high price means that engineers use it for only the most crucial designs. With the crystals, Altair engineers can quickly reduce titanium oxide to pure titanium metal.
This research is funded by the U.S. Defense Advanced Research Projects Agency (DARPA), whose leaders hope to use cheap titanium to improve the fuel consumption of tanks, humvees, and airplanes, while retaining their strength.
Finally, Altair engineers can also use nanomaterials as chemical and biological sensors. They are working with researchers at the Nanotechnology Research and Computation Center at Western Michigan University in Kalamazoo, Mich. The team is in the midst of a three-year program funded by a grant from the U.S. Department of Energy.
The researchers attach a layer of nanoparticles to a sensor surface, and then coat them with a synthesized oligomer-a simple type of chemical polymer.
“They sit there like crabs with their claws out to grab things,” Lyon says. “They can catch different types of chemicals depending on the kind of oligomer, then they react by changing the wavelength of light they reflect. It’s a lab on a chip.”
Portable lab
A chemist who wants to identify the components of a new substance uses a mass spectrometer, a delicate, expensive instrument in a laboratory. Now researchers at RTI International, a Department of Defense-funded nonprofit institute in Research Triangle Park, N.C., have found another option.
They use carbon nanotubes to make a micro mass spectrometer, says Ken Williams, director of RTI’s center for materials and electronics technologies.
Carbon nanotubes are hollow cylinders made of carbon atoms. Each tube has a diameter 10,000 times smaller than a human hair, making it useful as an electrical semiconductor with high strength and thermal conductivity.
Researchers use them to identify new compounds by finding an agent that reacts with each chemical or biological target. This micro mass spectrometer is lightweight and durable enough to be carried onto the battlefield by a foot soldier or loaded on an unmanned aerial vehicle (UAV).
RTI researchers are also working on ways to harness the optical properties of nanoparticles for applications in communications or solid-state lighting. Such a device could capture light at a certain wavelength and convert it to white light, saving a huge amount of energy and cost compared to fluorescent bulbs, Williams says.
Nano electricity
Conveying electricity to the solider in the field is one of the great challenges of modern warfare. Today’s troops use more electrical devices than ever before, and they travel from base camp to battlefield faster than engineers can string power lines.
Planners at the U.S. Defense Advanced Research Projects Agency (DARPA) think the solution could be solar cells built with nanoscale materials.
In February 2004, DARPA leaders granted $6 million to Konarka Technologies Inc. in Lowell, Mass., to study hybrid photovoltaic cells. The technology is crucial for military action since such cells could provide battery charging on the battlefield, remote power for soldiers and unmanned vehicles, and solar-powered sensor networks.
Konarka will share the award over five years with research and development partners including Arizona State University; National Renewable Energy Laboratory (NREL); University of Delaware; University of Massachusetts, Lowell; and U.S. Army Soldier Systems Center in Natick, Mass.
Engineers at Konarka create materials that absorb both sunlight and indoor light and convert them into electrical energy. To do it, they use nanomaterials to inject dye into titanium dioxide, a white pigment common in toothpaste and paint. The dye absorbs light, which travels through the titanium dioxide and a series of electrodes, becoming electrical energy.
Until now, photovoltaic cells have been built with glass or silicon, creating stiff, heavy panels. Konarka’s cells are built on flexible thin film and plastics. While the new cells are cheaper, they are not as efficient; Konarka’s devices operate at 8 to 10 percent efficiency, compared to 15 percent for traditional cells. The flexible cells make up the difference, however, in ease of use-users can deploy them anywhere, building solar cells into their tents, awnings, roofs, and windows. On the battlefield, they could generate electricity for lighting, sensing, communicating, and computing. The cells will soon become more efficient, company officials promise.
“Hybrid photovoltaic cells build on the breakthroughs we have already achieved with dye-sensitized cells and polymer cells. The hybrid cells will incorporate unique forms of polymers and semiconductors in the cells’ active layers,” says Russell Gaudiana, vice president of research and development for Konarka. “This funding will accelerate our development of hybrid cells that turn light into electricity with an estimated efficiency of more than 20 percent, which is a significant improvement over existing cells.”
Nanotechnology offers many other ways to move electricity to the battlefield. Researchers now use nanomaterials to improve the creation of electricity in power transformers, fuel cells, and solar cells. In July 2004, the Electricity Innovation Institute (E2I) and the Electric Power Research Institute (EPRI) awarded three Nanotechnology Research Grants for these topics, each worth $100,000.
The first winner, Craig Grimes at Pennsylvania State University, is seeking a way to fabricate e-nose sensors that would be installed on-line for the continuous monitoring of concentrations of eight gases in mineral-oil power transformers, to mitigate the risks and damage of power-transformer failure. His project is “Development of Nanotubular/Nanoporous Metal Oxide E-Nose Sensor for In-situ Monitoring of Dissolved Gases in Power Transformers.”
The second winners, Chien Wai and Frank Cheng at the University of Idaho, are trying to develop nanoscale electrocatalysts for highly efficient low-temperature fuel cells. They chose supercritical CO2, a nontoxic, nonflammable, and recyclable solvent. The project is called “Carbon Nanotube-Supported Catalytic Nanoparticles for Fuel Cell Applications.”
The third winner, Pamela Shapiro, also at the University of Idaho, is trying to use polymer synthesis to prepare quantum-dot solar cells, which would have three times the solar-energy conversion efficiency of current commercial technology. They rely on an ordered array of chalcopyrite (CuInS2) nanoparticles within an insulating polymer matrix. The project is called “Synthetic Approaches to New Photovoltaic Materials based on Ordered Chalcopyrite Quantum Dot Arrays in Polymer Matrices for the Development of High Efficiency Solar Cells.”