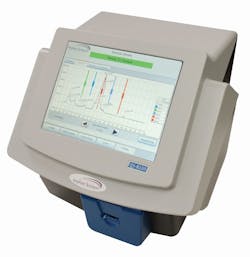
French airport adopts Implant Sciences explosives detection devices to protect travelers
WILMINGTON, Mass., 1 May 2015.Implant Sciences Corp. (OTCQB:IMSC), a manufacturer of explosives trace detection (ETD) for homeland security applications, has received an order for 25 QS-B220 explosives trace detection systems from a major airport in France. The systems will be used to fulfill the European security regulations requirement for screening passengers and checked bags.
"Our QS-B220 was selected for its superior performance in the airport operating environment. After conducting a long operational performance evaluation at the airport, the QS-B220's performance was demonstrated to be far superior to the competition. This, along with the fact that the QS-B220 is the only non-rad ETD to have both the original STAC (France's ETD approval) as well as the ECAC (European Civil Aviation Conference) qualification, drove the purchasing decision," says Dr. Darryl Jones, Implant Sciences' vice president of global sales and marketing.
The QS-B220 uses Ion Mobility Spectrometry (IMS) to detect and identify trace amounts of a wide variety of military, commercial, and homemade explosives as well as illicit drugs. With lower maintenance requirements than competing systems, the QS-B220 can be deployed for a much lower total cost of ownership than other approved products, officials say. Featuring a radioactive material-free design, push-button maintenance and diagnostics, and a patented inCal internal automatic calibration system, the QS-B220 desktop trace detection is designed to deliver ease of use.
Implant Sciences develops and manufactures advanced detection capabilities to counter and eliminate the ever-evolving threats from explosives and drugs. The company's ETDs have received approvals and certifications from several international regulatory agencies including the TSA in the U.S., ECAC in Europe, CAAC and the Ministry of Public Safety in China, Russia FSB, STAC in France, and the German Ministry of the Interior.